Atomic Number
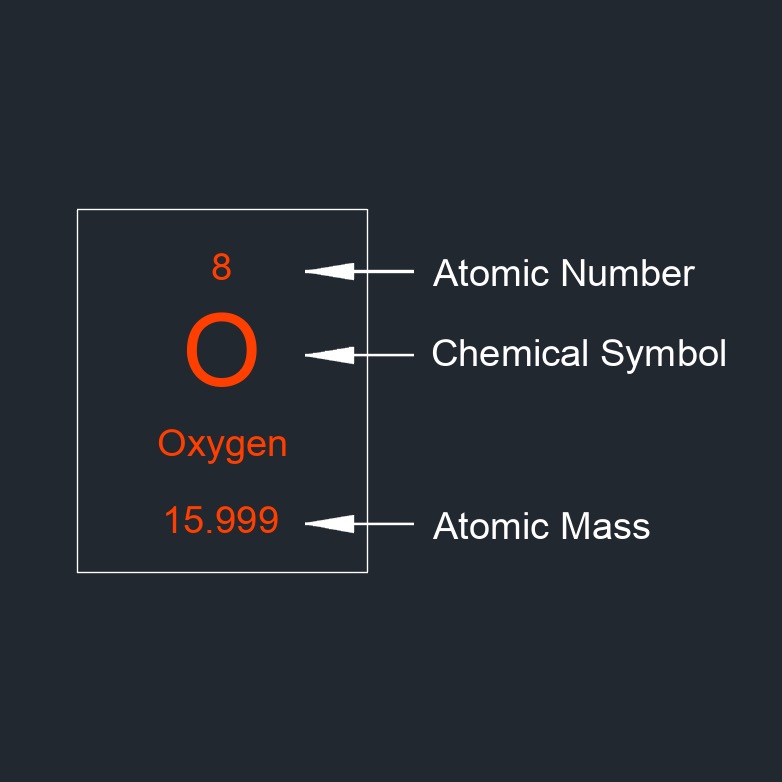 Atomic number, abbreviated as Z, a dimensionless number, is equal to the number of protons in an atom's nucleus. The atomic number determines which element an atom is. The number of protons in the nucleus of an atom is equal to the number of electrons in the neutral atom, so the atomic number also tells us the number of electrons in a neutral atom of that element.
Atomic number, abbreviated as Z, a dimensionless number, is equal to the number of protons in an atom's nucleus. The atomic number determines which element an atom is. The number of protons in the nucleus of an atom is equal to the number of electrons in the neutral atom, so the atomic number also tells us the number of electrons in a neutral atom of that element.
Each element has a unique atomic number, which is used to arrange the elements in the periodic table. The atomic number of an element is also used to determine the isotopes of that element, which have the same number of protons but different numbers of neutrons in the nucleus. The atomic number plays a significant role in determining the chemical properties and behavior of an element. Elements with different atomic numbers have distinct chemical properties and reactivity due to variations in their electron configurations and atomic structures.

