Condensation
 Condensation is the process by which a substance changes its state from a gas to a liquid as a result of losing heat. This occurs when a gas is cooled to its dew point, which is the temperature at which the gas begins to form liquid droplets. When a gas is cooled, the molecules or atoms within it lose kinetic energy and move more slowly. As the temperature drops, the intermolecular forces between the particles become strong enough to overcome the kinetic energy and hold the particles in a more ordered structure, resulting in the formation of a liquid.
Condensation is the process by which a substance changes its state from a gas to a liquid as a result of losing heat. This occurs when a gas is cooled to its dew point, which is the temperature at which the gas begins to form liquid droplets. When a gas is cooled, the molecules or atoms within it lose kinetic energy and move more slowly. As the temperature drops, the intermolecular forces between the particles become strong enough to overcome the kinetic energy and hold the particles in a more ordered structure, resulting in the formation of a liquid.
Condensation is an important process in many natural and industrial settings. In the atmosphere, it is responsible for the formation of clouds, fog, and dew. In refrigeration and air conditioning systems, it is used to remove heat from the air and cool the surrounding environment. In chemical and biological processes, it is used to separate and purify substances.
The dew point is a physical property that is specific to each substance and is dependent on factors such as pressure and humidity. The dew point can be used as a tool to predict when and where condensation will occur, and to control the conditions under which it occurs.
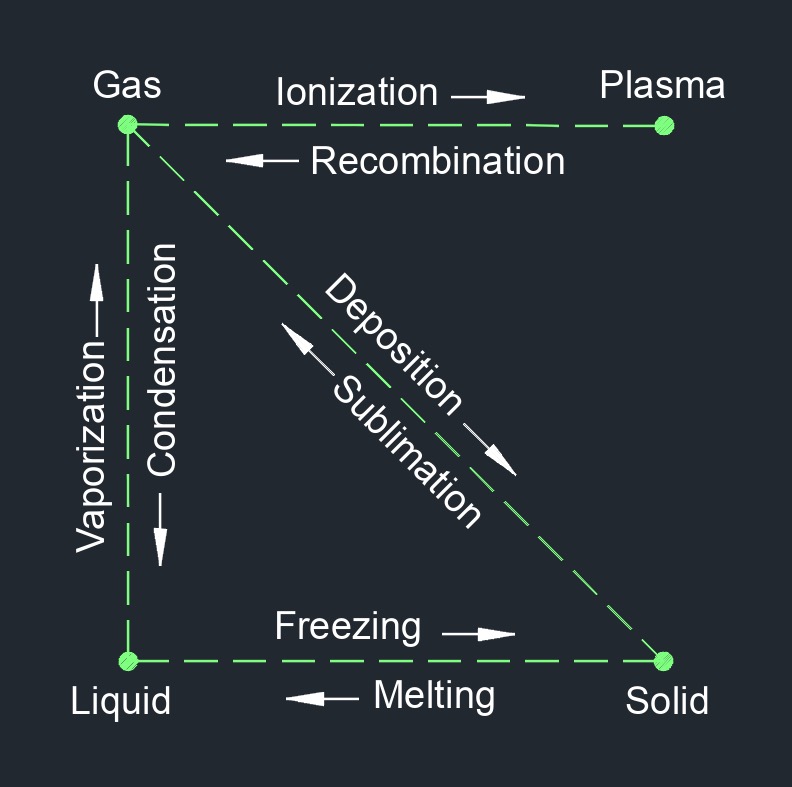
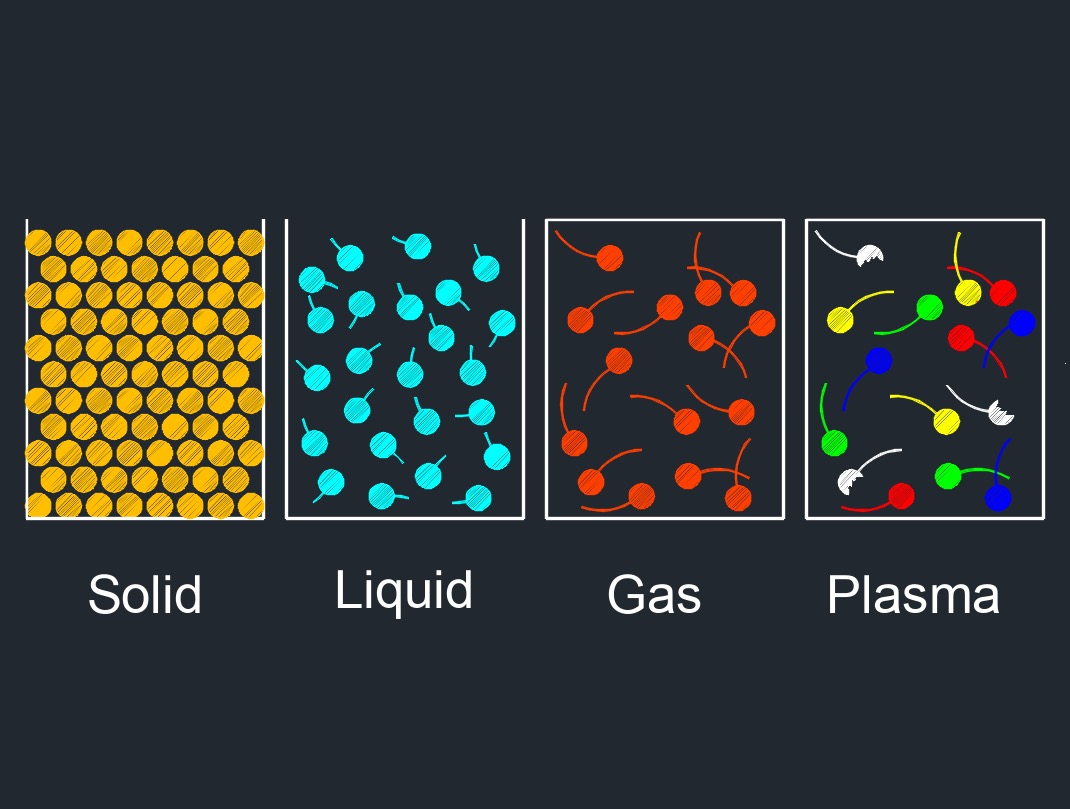
There are four phases of matter: gas, liquid, plasma, and solid.

