Molarity
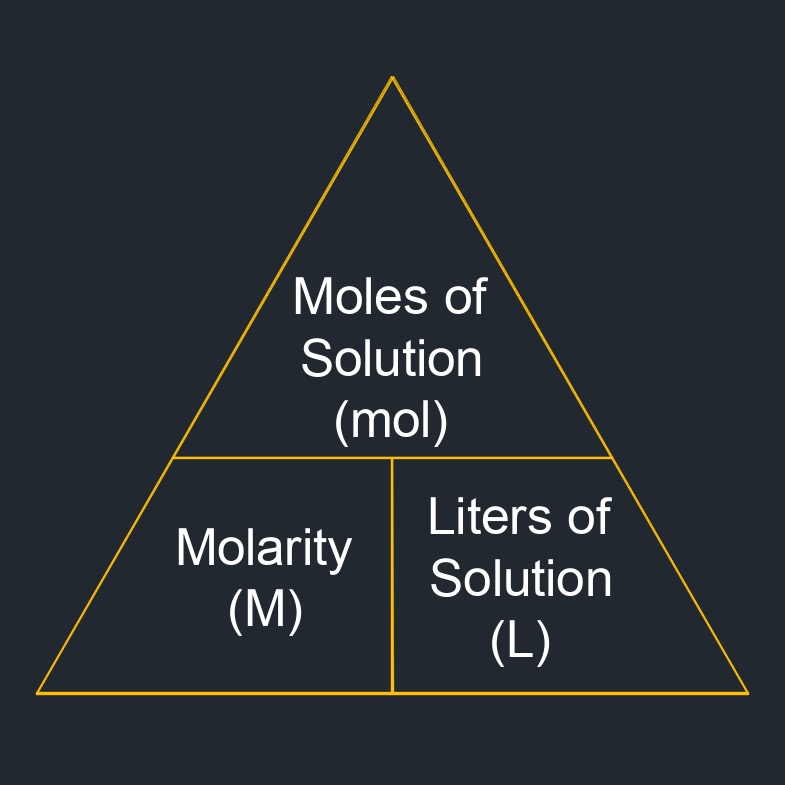
Molarity Formula |
||
| \( M \;=\; \dfrac{ n }{ V } \) | ||
| Symbol | English | Metric |
| \( M \) = Molarity | \(mol\;/\;gal\) | \(mol\;/\;L\) |
| \( n \) = Number of Moles of Solution | \(dimensionless\) | \(dimensionless\) |
| \( V \) = Solution Volume | \(gal\) | \(L\) |
 Molarity, abbreviated as M, is a measure of the concentration of a solute in a solution. Molarity is a basic concept in chemistry and is used extensively in various calculations, including dilutions, stoichiometry, and determining reaction rates. It provides a convenient way to express the concentration of a solution in a quantitative manner.
Molarity, abbreviated as M, is a measure of the concentration of a solute in a solution. Molarity is a basic concept in chemistry and is used extensively in various calculations, including dilutions, stoichiometry, and determining reaction rates. It provides a convenient way to express the concentration of a solution in a quantitative manner.

