Boyle's Law
Boyle's Law Formula |
||
|
\( p_1 \cdot V_1 \;=\; p_2 \cdot V_2 \) (Boyle's Law) \( p_1 \;=\; \dfrac{ p_2 \cdot V_2 }{ V_1 } \) \( V_1 \;=\; \dfrac{ p_2 \cdot V_2 }{ p_1 } \) \( p_2 \;=\; \dfrac{ p_1 \cdot V_1 }{ V_2 } \) \( V_2 \;=\; \dfrac{ p_1 \cdot V_1 }{ p_2 } \) |
||
| Symbol | English | Metric |
| \( p_1 \) = Initial Pressure | \(lbf\;/\;in^2\) | \(N\;/\;m^2\) |
| \( V_1 \) = Initial Volume | \( ft^3 \) | \( m^3 \) |
| \( p_2 \) = Final Pressure | \(lbf\;/\;in^2\) | \(N\;/\;m^2\) |
| \( V_2 \) = Final Volume | \( ft^3 \) | \( m^3 \) |
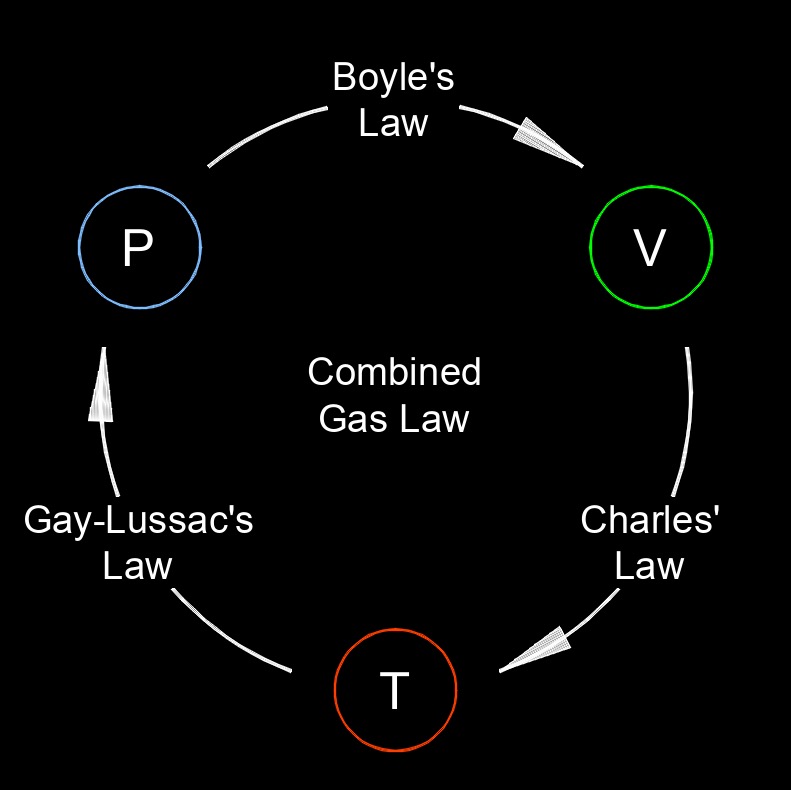 Boyle's law, is one of the fundamental principles in the field of gas physics and thermodynamics. It describes the relationship between the pressure and volume of a gas when the temperature and the amount of gas are held constant. This means that if you decrease the volume of a gas while keeping the temperature and the amount of gas constant, the pressure will increase. Conversely, if you increase the volume, the pressure will decrease. In simple terms, as the volume of a gas decreases, the gas particles are crowded into a smaller space, leading to more frequent collisions with the container walls, which increases the pressure.
Boyle's law, is one of the fundamental principles in the field of gas physics and thermodynamics. It describes the relationship between the pressure and volume of a gas when the temperature and the amount of gas are held constant. This means that if you decrease the volume of a gas while keeping the temperature and the amount of gas constant, the pressure will increase. Conversely, if you increase the volume, the pressure will decrease. In simple terms, as the volume of a gas decreases, the gas particles are crowded into a smaller space, leading to more frequent collisions with the container walls, which increases the pressure.
Boyle's Law is an important principle in understanding the behavior of gases and is often applied in various scientific and practical applications, including in the design of pressure vessels, scuba diving, and gas storage.

