Deposition
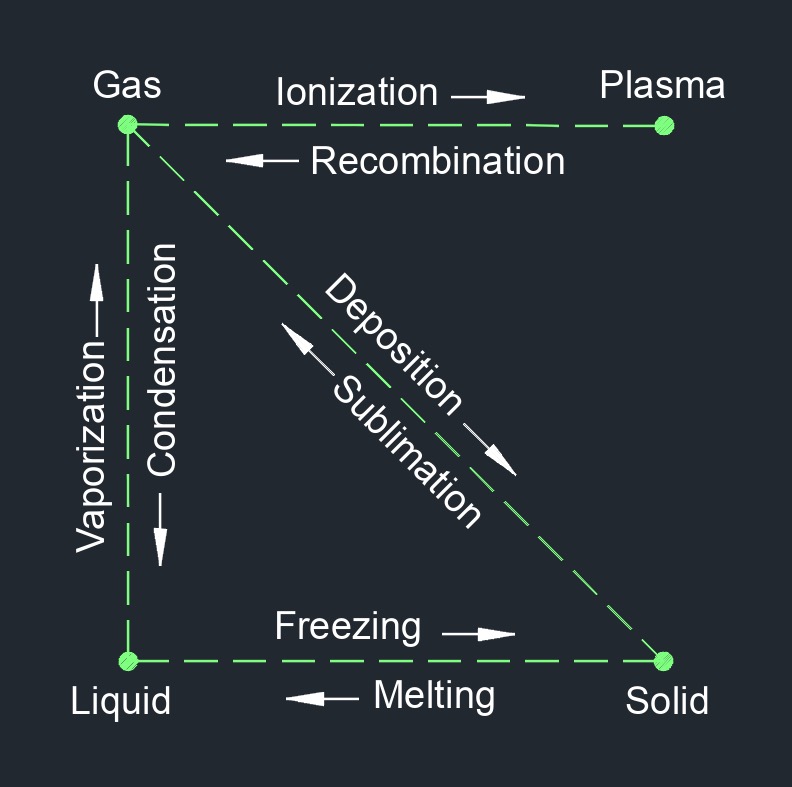
 Deposition is a process by which a substance transitions directly from a gaseous state to a solid state without passing through a liquid phase. It is the opposite of sublimation, which is the process of transitioning from a solid to a gas phase without passing through a liquid phase. Deposition occurs when a gas loses heat energy and the molecules lose kinetic energy, causing them to slow down and come together to form a solid. This process is most commonly observed in the formation of frost or ice on surfaces when water vapor in the air comes into contact with a surface that is below freezing point.
Deposition is a process by which a substance transitions directly from a gaseous state to a solid state without passing through a liquid phase. It is the opposite of sublimation, which is the process of transitioning from a solid to a gas phase without passing through a liquid phase. Deposition occurs when a gas loses heat energy and the molecules lose kinetic energy, causing them to slow down and come together to form a solid. This process is most commonly observed in the formation of frost or ice on surfaces when water vapor in the air comes into contact with a surface that is below freezing point.
Deposition is also an important process in various industrial applications such as thin film deposition, where a solid coating is deposited on a surface through the condensation of a vapor or gas, and chemical vapor deposition, where a solid film is formed on a substrate through the deposition of a reactive gas or vapor. In geology, deposition refers to the accumulation of sedimentary material such as sand, mud, or organic matter on the Earth's surface, typically by the action of water, wind, or ice. This process is important in the formation of various geological features such as sedimentary rocks, deltas, and sand dunes.
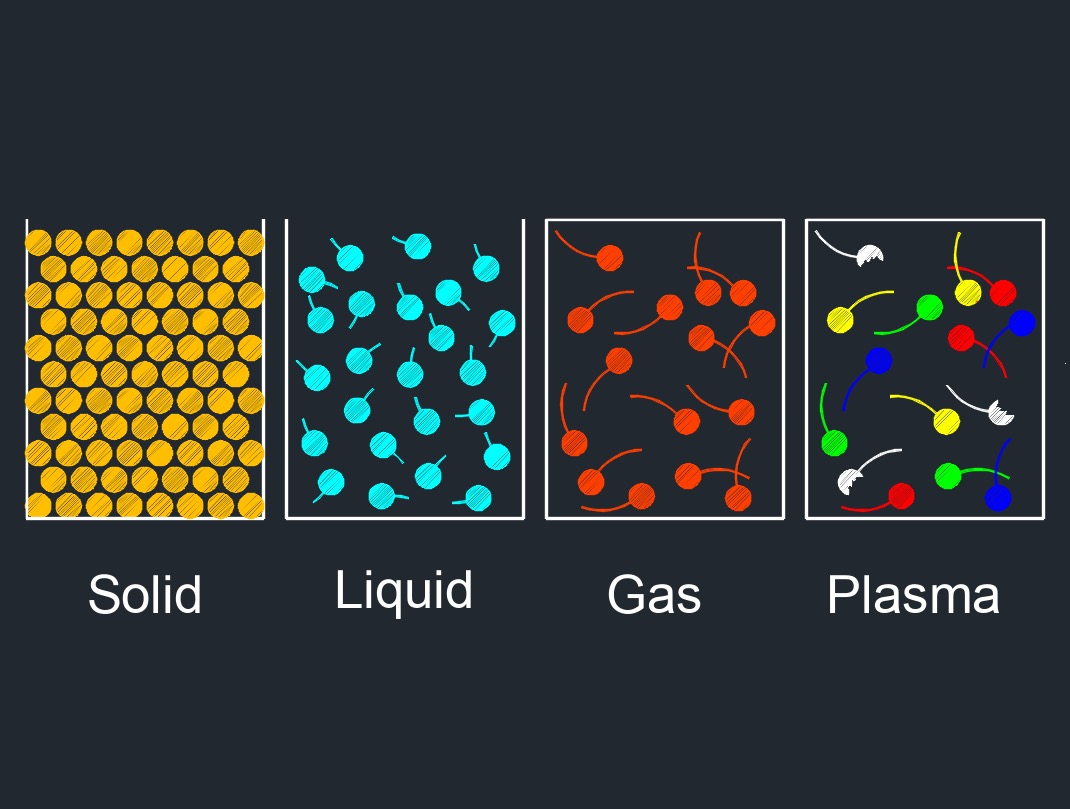
There are four phases of matter: gas, liquid, plasma, and solid.

