Ideal Gas Law
Ideal Gas Law Formula
|
||
|
\( p \cdot V \;=\; n \cdot R \cdot T \) (Ideal Gas Law) \( p \;=\; \dfrac{ n \cdot R \cdot T }{ V }\) \( V \;=\; \dfrac{ n \cdot R \cdot T }{ p }\) \( n \;=\; \dfrac{ p \cdot V }{ R \cdot T }\) \( R \;=\; \dfrac{ p \cdot V }{ n \cdot T }\) \( T \;=\; \dfrac{ p \cdot V }{ n \cdot R }\) |
||
| Symbol | English | Metric |
| \( p \) = Gas Pressure | \(lbf\;/\;in^2\) | \(Pa\) |
| \( V \) = Gas Volume | \( in^3 \) | \( mm^3 \) |
| \( n \) = Number of Moles of the Gas | \(dimensionless\) | \(dimensionless\) |
| \( R \) = Specific Gas Constant (Gas Constant) | \(ft-lbf\;/\;lbm-R\) | \(J\;/\;kg-K\) |
| \( T \) = Gas Temperature | \(^\circ R \) | \(^\circ K \) |
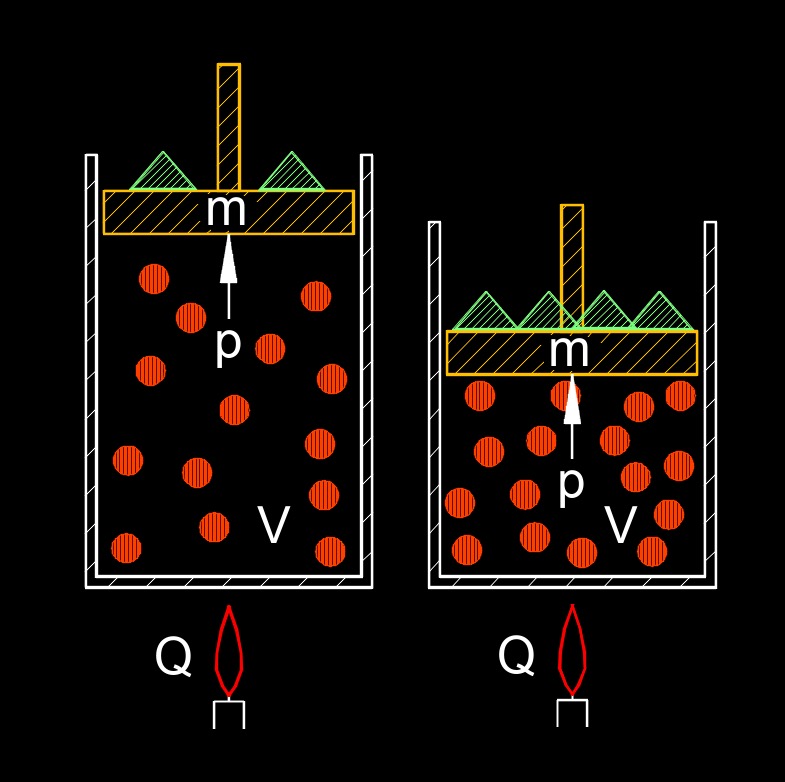 Ideal gas law, also called general gas equation, can be used to predict pressure, temperature, and volume changes in ideal gasses. An ideal gas is defined as one in which all collisions between atoms or molecules are perfectly elastic and in which there are no intermolecular attractive forces. In the real world, the ideal gas law must be corrected but it can serve as a good approximation for initial calculations. The ideal gas law states that for a given amount of gas, the product of its pressure and volume is directly proportional to the number of moles of the gas and the absolute temperature.
Ideal gas law, also called general gas equation, can be used to predict pressure, temperature, and volume changes in ideal gasses. An ideal gas is defined as one in which all collisions between atoms or molecules are perfectly elastic and in which there are no intermolecular attractive forces. In the real world, the ideal gas law must be corrected but it can serve as a good approximation for initial calculations. The ideal gas law states that for a given amount of gas, the product of its pressure and volume is directly proportional to the number of moles of the gas and the absolute temperature.
The ideal gas law can be used to calculate various properties of ideal gases, such as finding the unknown pressure, volume, temperature, or number of moles if the other variables are known. It is widely used in fields such as chemistry, physics, and engineering to analyze and predict the behavior of gases under different conditions. It's important to note that the ideal gas law assumes idealized gas behavior, where the gas particles are considered point masses with no intermolecular forces and occupy negligible volume. While real gases may deviate from ideal behavior under certain conditions, the ideal gas law is a useful approximation for many practical applications.

