Vapor
Vapor Quality formula |
||
|
\( x \;=\; m_{vapor} \;/\; m_{liquid} + m_{vapor} \) (Vapor Quality) \( x < 1 \; \rightarrow \) (wet steam) \( x > 1 \; \rightarrow \) (dry steam) |
||
| Symbol | English | Metric |
| \( x \) = Steam Quality | \(dimensionless\) | \(dimensionless\) |
| \( m_{liquid} \) = Liquid Mass | \(lbm\) | \(kg\) |
| \( m_{vapor} \) = Vapor Mass | \(lbm\) | \(kg\) |
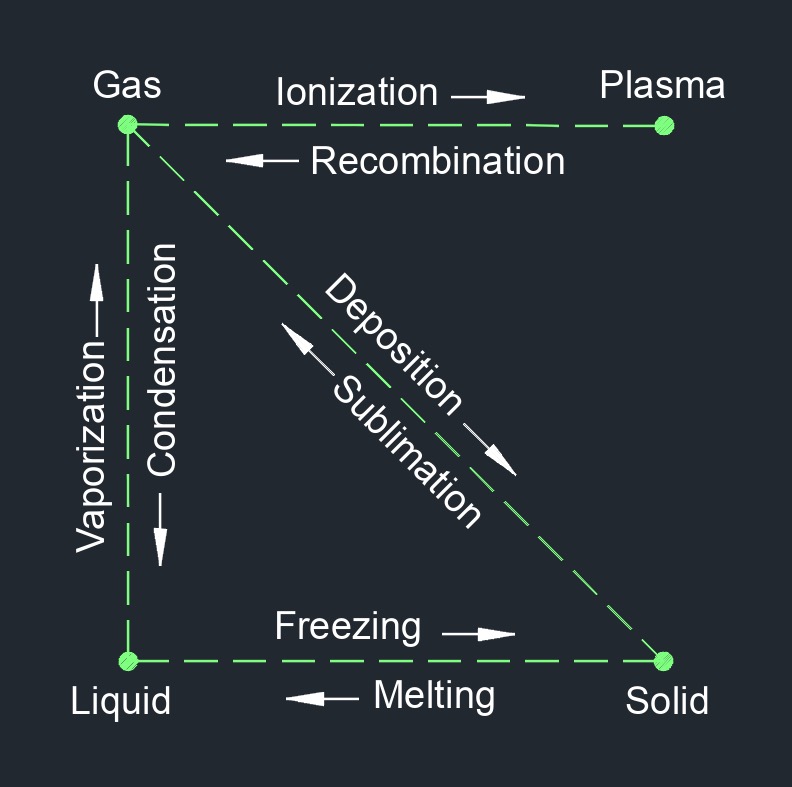
Vapor is a gaseous state of matter that exists at or below its critical temperature and pressure. It is a gas that is generated from a liquid or a solid by heating or by reducing the pressure on the substance, causing its molecules to escape into the air.
The term "vapor" is often used interchangeably with "gas", but it specifically refers to the gaseous form of a substance that is normally a liquid or solid at standard temperature and pressure. Examples of vapor include water vapor (the gaseous form of water), steam (the gaseous form of water that has been heated to its boiling point), and gasoline vapor (the gaseous form of gasoline).
Vapor can be produced through various processes such as evaporation, boiling, sublimation, and vaporization. The behavior of vapor is influenced by various factors such as temperature, pressure, and the presence of other substances in the surrounding environment. Vapor is important in many fields such as meteorology, where water vapor plays a significant role in weather patterns and the formation of clouds and precipitation. It is also important in many industrial processes such as distillation, where the separation of different substances is achieved through their different boiling points and vapor pressures.

