Saturated Vapor
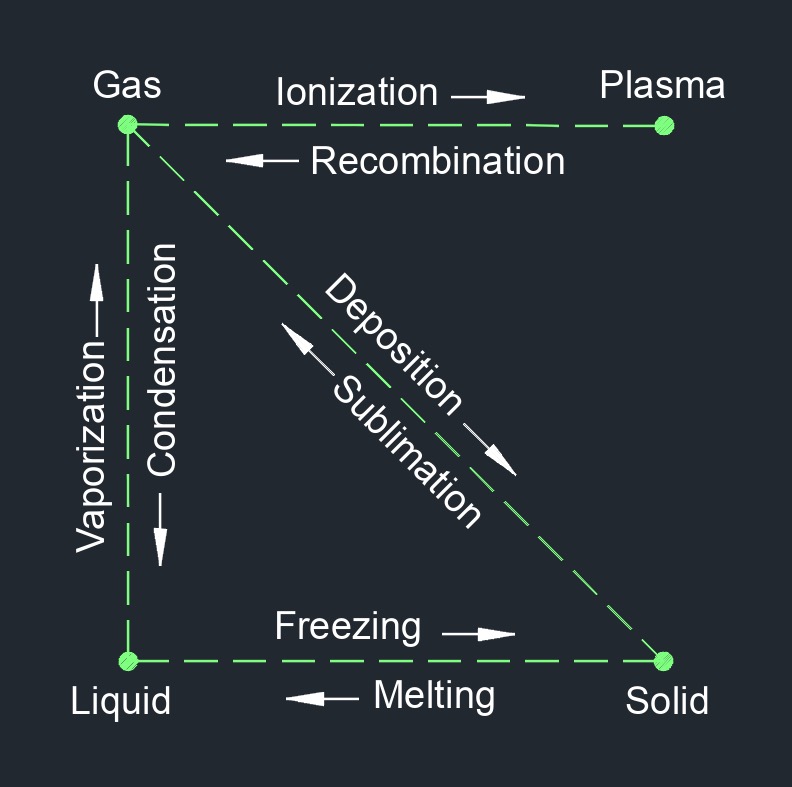
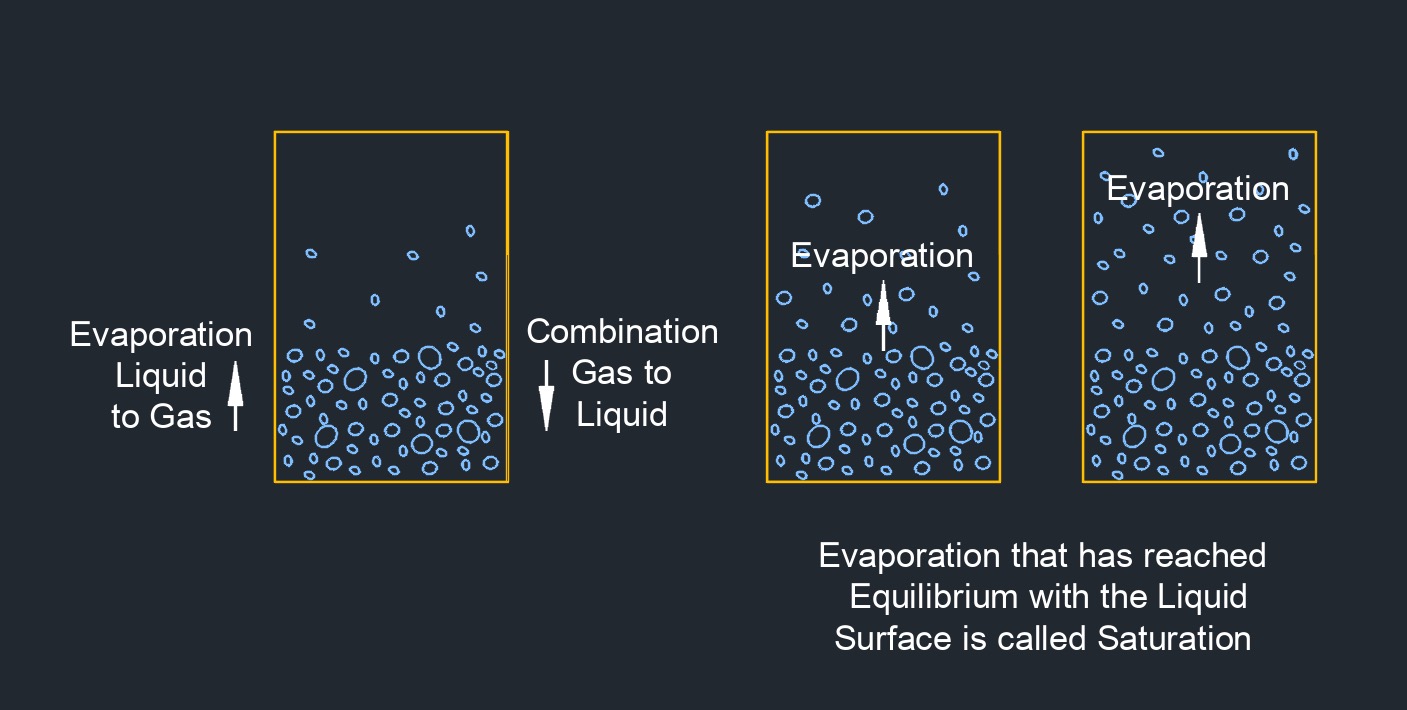 Saturated vapor is a vapor or gas that is in equilibrium with its own liquid phase at a specific temperature and pressure. At this point, the vapor has reached its maximum possible concentration of vapor molecules in the gas phase and any further addition of heat or pressure will cause the gas to become superheated or to condense into its liquid phase, respectively. The concept of saturated vapor is closely related to the concept of vapor pressure, which is the pressure exerted by a vapor in equilibrium with its liquid phase at a given temperature. At the vapor pressure, the rate of evaporation of the liquid is equal to the rate of condensation of the vapor, resulting in a stable equilibrium.
Saturated vapor is a vapor or gas that is in equilibrium with its own liquid phase at a specific temperature and pressure. At this point, the vapor has reached its maximum possible concentration of vapor molecules in the gas phase and any further addition of heat or pressure will cause the gas to become superheated or to condense into its liquid phase, respectively. The concept of saturated vapor is closely related to the concept of vapor pressure, which is the pressure exerted by a vapor in equilibrium with its liquid phase at a given temperature. At the vapor pressure, the rate of evaporation of the liquid is equal to the rate of condensation of the vapor, resulting in a stable equilibrium.
Examples of saturated vapor include water vapor in the atmosphere, which can condense to form clouds or fog when the temperature drops, and the steam produced by boiling water at a constant temperature and pressure. Saturated vapor is important in various fields such as thermodynamics, chemistry, and engineering. Understanding the behavior of saturated vapor is essential for designing and operating systems that involve vapor liquid equilibrium, such as distillation columns, refrigeration systems, and power generation facilities.

